MENU

|
| The FNCA FY2009 Workshop on Radiation Oncology |
Report of
FNCA FY2009 Workshop on Radiation Oncology
January, 18(Mon)-21(Thu), 2010
Kuching, Sarawak, Malaysia
The FNCA FY2009 Workshop on Radiation Oncology was held from January 18 to 21, 2010, in Kuching, Sarawak, Malaysia. The meeting was organized by Ministry of Health, Malaysia and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, in cooperation with Nuclear Safety Research Association (NSRA) of Japan. Seventeen Representatives from 9 FNCA Member States, namely Bangladesh, China, Indonesia, Japan, Korea, Malaysia, the Philippines, Thailand and Vietnam, participated in the workshop.
This project carried out some international joint clinical studies in order to establish common treatment protocols for uterine CERVIX cancer and nasopharyngeal cancer, which affect large numbers of people in FNCA member countries, and finally to improve the technique of radiation Oncology in the Asian region.
 |
| The participants of Workshop |
Phase II Study of Chemoradiotherapy for Locally Advanced Cervical Cancer (CERVIX-III)The recent clinical data of Phase II study of concurrent chemoradiotherapy (CCRT) for locally advanced cervical cancer (CERVIX-III) was presented by representatives of each participating country (China reported on 18 patients, Indonesia 5, Japan 32, Korea 10, Malaysia 14, Philippines 12, Thailand 19, and Vietnam 10). Total of 120 patients with Stage IIB (60pts) and Stage IIIB (60pts) diseases were registered and analyzed. The incidence and severity of acute toxicity were within acceptable level and most late complications were mild or moderate on the basis of the 4-year follow-up rate of 94%. The 4-year rates of grade 3-4 late rectal and bladder complications were 6.7% and 0%, respectively. The 4 year over all survival and local control rates of the patients were 60.4 % and 78.4%, respectively. CERVIX-III results were comparable or superior to those of the recognized international clinical reports. This demonstrated that the concurrent CRT was feasible and effective for patients with locally advanced cervical cancer in FNCA countries. And actually this protocol has been rather disseminated as the standard one for locally advanced uterine CERVIX cancer there. The follow-up was recommended to continue for at least 5 years.
Phase II Study of Concurrent Chemotherapy and Extended-Field Radiotherapy for Locally Advanced Cervical Cancer (CERVIX-IV)The recent clinical data of Phase II Study of Concurrent Chemotherapy and Extended-Field Radiotherapy for Locally Advanced Cervical Cancer (CERVIX-IV) was presented by representatives of each participating country (Bangladesh reported on 2 patients, China 3, Indonesia 8, Japan 8, Korea 7, Malaysia 1, Philippines 4 , Thailand 1, and Vietnam 0). The 1-year local control rate was 100% and the 1-year progression-free survival rate was 83%. It was decided that modified protocol presented last year will be continued in view of the lesser toxicity observed. Additional patient enrollment is encouraged and longer follow-up is needed to evaluate the treatment safety and efficacy.
Phase II Study of Chemoradiotherapy for NPC (TxN2-3) (NPC-I)Registration was closed in 2009. The total number of patients was 128.The recent clinical data of Phase II Study of Chemoradiotherapy for NPC (TxN2-3) (NPC-I) was presented by representatives of each participating country. (Bangladesh reported on 1 patient, China 5, Indonesia 5, Japan 0, Korea 5, Malaysia 25, Philippines 5, Thailand 5, and Vietnam 82). The FNCA study showed a decrease in overall survival compared to other studies. It was suggested that Subgroup analysis should be done based on N2, N3, and bone scan. In addition, we will need a control arm of patients treated with radiotherapy alone. Further follow-up is needed to confirm long-term efficacy and late toxicities.
Phase II Study of Chemoradiotherapy for NPC (T3-4N0-3) ( NPC-II)The total number of registered patients was 59. The recent clinical data of Phase II Study of Chemoradiotherapy for NPC (T3-4 N0-1) (NPC-II) with 59 patients was presented by representatives of each participating country. (Bangladesh reported on 0 patient, China 0, Indonesia 12, Japan 0, Korea 0, Malaysia 6, Philippines 0, Thailand 1, and Vietnam 40). 76 percent of patients completed 6 cycles of chemotherapy. Actual mean dose of cisplatin (165mg/m2) was slightly lower than Chan's study (174-187 mg/m2). Incidence of nausea, vomiting and leukopenia were also lower. Toxicities were manageable with only a few patients who developed Grade IV toxicities. Clearance rate (CR) was 96 %, which was a favorable response for T3-4 disease. With a median follow-up time of 36 months, the overall 3 year survival rate was 80%.
Registration of new patients was suggested. Meanwhile, further follow-up is needed to confirm long-term efficacy and late toxicities.
QA/QC of Radiation DosimetryReport on intercomparison measurements using glass dosimeters done at 4 centers in 4 countries on 14 photon beams were presented. Every 14 beams were within optimum level. Regarding the schedule of the next field work, further work remains to be done in Bangladesh and Vietnam (Hanoi).
Open LectureOpen lecture was held at Sarawak General Hospital. Six lectures on Rheumatology and Radiation therapy were given by the staff of Sarawak General Hospital and the participants of the workshop. There were 64 participants including radiation oncologists, medical physicists, radiotherapy technicians, nurses, medical doctors from other departments, and medical students.
|
Minutes of
FNCA FY2009 Workshop on Radiation Oncology
January, 18(Mon)-21(Thu), 2010
Kuching, Sarawak, Malaysia
(1) Following the agreement of the 10th Forum for Nuclear Cooperation in Asia (FNCA) Coordinators Meeting in March 2009, and the 10th FNCA Meeting in December 2009, the FNCA FY2009 Workshop on Radiation Oncology was held from January 18 to 21, 2010, in Kuching, Sarawak, Malaysia. The meeting was organized by Ministry of Health, Malaysia and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, in cooperation with Nuclear Safety Research Association (NSRA) of Japan. Representatives from 9 FNCA Member States, namely Bangladesh, China, Indonesia, Japan, Korea, Malaysia, the Philippines, Thailand and Vietnam, participated in the workshop.
Opening ceremony(2) Dr. Zulkipli Jantan, Sarawak State Health Director opened the Workshop officially with his opening remarks on behalf of host country. He stressed the importance of this project in the frame of sciences and technologies. Dr. Hirohiko Tsujii, Project Leader of Japan, made his remarks and explained the objectives of the meeting and the outline of the program.
(3) Dr. Tang Tieng Swee gave a Special Lecture "Present Status of Radiation Oncology in Malaysia". He explained the history and present status of radiation oncology in Malaysia.
(4) The agenda was adopted, and chairpersons and reporters were elected. (See Annex 1) All the participants were introduced.
Session 1: Phase II Study of Chemoradiotherapy for Locally Advanced Cervical Cancer (CERVIX-III)(5) The recent clinical data of Phase II study of concurrent chemoradiotherapy (CCRT) for locally advanced cervical cancer (CERVIX-III) was presented by representatives of each participating country (China reported on 18 patients, Indonesia 5, Japan 32, Korea 10, Malaysia 14, Philippines 12, Thailand 19, and Vietnam 10). The summary of the follow-up data was presented by Dr. Shingo Kato. Total of 120 patients with Stage IIB (60pts) and Stage IIIB (60pts) diseases were registered and analyzed. The incidence and severity of acute toxicity were within acceptable level and most late complications were mild or moderate on the basis of the 4-year follow-up rate of 94%. The 4-year rates of grade 3-4 late rectal and bladder complications were 6.7% and (0)%, respectively. The 4 year over all survival and local control rates of the patients were 60.4 % and 78.4%, respectively. There was also interesting discussion about the failure pattern. The sites of failure are 18% for local site and 34% for distant metastases. The main sites of failures for distant metastases are para-aortic lymph nodes in 17 patients and supraclavicular nodes in 10 patients. CERVIX-III results were comparable or superior to those of the recognized international clinical reports. This demonstrated that the concurrent CRT was feasible and effective for patients with locally advanced cervical cancer in FNCA countries. The relationship between rectal and bladder doses and late toxicities were evaluated. The follow-up was recommended to continue for at least 5 years.
Session 2: Phase II Study of Concurrent Chemotherapy and Extended-Field Radiotherapy for Locally Advanced Cervical Cancer (CERVIX-IV)(6) The recent clinical data of Phase II Study of Concurrent Chemotherapy and Extended-Field Radiotherapy for Locally Advanced Cervical Cancer (CERVIX-IV) was presented by representatives of each participating country (Bangladesh reported on 2 patients, China 3, Indonesia 8, Japan 8, Korea 7, Malaysia 1, Philippines 4 , Thailand 1, and Vietnam 0). Dr. Shingo Kato presented the summary of the clinical data. The 1-year local control rate was 100% and the 1-year PFS was 83%. However, the number of registered patients is still small. Additional patient enrollment is encouraged and longer follow-up is needed to evaluate the treatment safety and efficacy.
(7) An open discussion on the clinical data of CERVIX-IV followed. The difficulties encountered in patient registration are the required CT Scan of the abdomen and pelvis, the toxicities when the pelvis and para-aortic regions are treated simultaneously and the cost of treatment. It was decided that modified protocol presented last year will be continued in view of the lesser toxicity observed. Weekly cycles of chemotherapy may be between 5-6. Overall treatment time should not be more than 60 days. The data centre will provide each participating country with the modified treatment protocol by April, 2010.
(8) An open discussion on the next clinical trial on cervical cancer was also made. Several on-going clinical trials were presented by Dr. S. Kato and its feasibility to be conducted in FNCA countries was discussed. Dr. Cho from Korea was requested to present his countries data on the use of CDDP 75 mg/m2 given every 3 weeks on the next FNCA meeting.
Session 3: QA/QC of External Beam Therapy(9) Report on intercomparison measurements using glass dosimeters done at 4 centers in 4 countries on 14 photon beams were presented by Dr. Hideyuki Mizuno. Every 14 beams were within optimum level.
(10) Regarding the schedule of the next field work, further work remains to be done in Bangladesh and Vietnam (Hanoi).
(11) Dr. Hideyuki Mizuno presented work in progress for survey of status medical physicists in FNCA countries and updated all missing data during the presentation.
Session 4: Phase II Study of Chemoradiotherapy for NPC (TxN2-3) (NPC-I)(12) The recent clinical data of Phase II Study of Chemoradiotherapy for NPC (TxN2-3) (NPC-I) was presented by representatives of each participating country. (Bangladesh reported on 1 patient, China 5, Indonesia 5, Japan 0, Korea 5, Malaysia 25, Philippines 5, Thailand 5, and Vietnam 82). Dr. Tatsuya Ohno presented the summary and the evaluation of the FNCA NPC-I study results, showing comparisons with different studies. He highlighted that the FNCA study showed a decrease in overall survival compared to other studies. Hence, there was a discussion to refine the present NPC I protocol. Subgroup analysis should be done based on N2, N3, and bone scan. In addition, we will need a control arm of patients treated with radiotherapy alone.
(13) Registration was closed in 2009. The total number of patients was 128. Further follow-up is needed to confirm long-term efficacy and late toxicities.
Session 5: Phase II Study of Chemoradiotherapy for NPC (T3-4 N0-1) (NPC-II)(14) The recent clinical data of Phase II Study of Chemoradiotherapy for NPC (T3-4 N0-1) (NPC-II) with 59 patients was presented by representatives of each participating country. (Bangladesh reported on 0 patient, China 0, Indonesia 12, Japan 0, Korea 0, Malaysia 6, Philippines 0, Thailand 1, and Vietnam 40). Dr. Tatsuya Ohno presented the summary and the evaluation of the FNCA NPC-II study results. 76 percent of patients completed 6 cycles of chemotherapy. Actual mean dose of cisplatin (165mg/m2) was slightly lower than Chan's study (174-187 mg/m2). Incidence of nausea, vomiting and leukopenia were also lower. Toxicities were manageable with only a few patients who developed Grade IV toxicities. Clearance rate (CR) was 96 %, which was a favorable response for T3-4 disease. With a median follow-up time of 36 months, the overall 3 year survival rate was 80%.
(15) The total number of registered patients was 59. Registration of new patients was suggested. Meanwhile, further follow-up is needed to confirm long-term efficacy and late toxicities.
(16) In the open discussion, Dr. Ohno suggested to analyze the T3 T4 subgroup of NPC I data and compare it with NPC II data in terms of local control of the disease.
Session 6: Technical Visit at Radiotherapy Division, Sarawak General Hospital(17) The participants conducted a Technical Visit to Sarawak General Hospital and observed the Radiotherapy Division. All the participants appreciated highly the set-up of the department. The successful Pain Management Program was shared with all the participants.
Session 7: Future plans, Other activities(18) There was discussion on the different cancers where radiotherapy was part of the treatment. No consensus was reached. Further survey was recommended.
(19) The initial results of NPC-I will be submitted to international journal for publication. Follow up will be continued so that we can have 5 year results.
(20) NPC III will be piloted with Conventional Technique or IMRT. The chemotherapy arm using cisplatin and 5FU (2-3cycles) will be used as neo-adjuvant regimen.
(21) Acceptability of FNCA protocols to member countries is shown in Annex 2.
(22) The participants suggested Japan as the host for the next workshop subject to an agreement by the Government of Japan. The tentative schedule is November, 2010.
(23) The importance to present the results in national and international meetings was emphasized.
Session 8: Drafting the Workshop Minutes(24) The draft of the minutes of the meeting was presented, discussed, amended and adopted.
Closing ceremony(25) Participants expressed appreciation to the local organizing committee, especially to Dr. Tang Tieng Swee and Dr. C.R. Beena Devi, for the excellent conduct of the Workshop and for their hospitality, to Dr. Hirohiko Tsujii for his dynamic leadership, and to NSRA. The participants especially expressed their gratitude for the continuing support from MEXT of Japan for this project. Closing Remarks were given by Dr. Takehiro Inoue on behalf of Dr. Hirohiko Tsujii, Japan, who expressed his appreciation to the host institute and the participants as well as his expectations for the future of the project. Dr. Muhd Noor B. Muhd Yunus, Deputy Director General (Technical), Malaysian Nuclear Agency (Nuclear Malaysia), closed the workshop officially.
Session 9: Open Lecture(26) Open lecture was held at Sarawak General Hospital, as a part of the workshop. On behalf of Dr. Hirohiko Tsujii, Dr. Takehiro Inoue gave opening remarks and Dr. Junaidi Diki, Director, Sarawak General Hospital, gave a Welcome Address. It was followed by the open lectures by 5 speakers. (Program attached in Annex 1). There were 64 participants including radiation oncologists, medical physicists, radiotherapy technicians, nurses, medical doctors from other departments, and medical students.
Annex 2 : Acceptability of FNCA Protocol to Participating Countries
| Country |
NPC I |
NPC II |
CX I |
CX II |
CX III |
CX IV |
| Bangladesh |
 |
 |
 |
- |
 |
 |
| China |
 |
 |
 |
- |
 |
 |
| Indonesia |
 |
 |
 |
 |
 |
 |
| Japan |
 |
 |
 |
- |
 |
 |
| Korea |
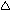 |
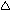 |
 |
 |
 |
 |
| Malaysia |
 |
 |
 |
- |
 |
 |
| Philippines |
 |
 |
 |
 |
 |
 |
| Thailand |
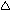 |
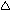 |
 |
 |
 |
 |
| Vietnam |
 |
 |
 |
- |
 |
 |
 = Acceptable, = Acceptable,  = Not Fully Acceptable = Not Fully Acceptable
Impact of FNCA Protocol Studies in member countries
Bangladesh
In Bangladesh, according to WHO Survey, about >200,000 new cancer patients are diagnosed each year. Oncology centres are inadequate. So, waiting time for patients are very long in government hospitals. Neoadjuvant chemotherapy is used in many cases due to delays in treatment. Concurrent chemoradiation is a preferred way of treatment for advanced NPC and Cervical Cancer in Bangladesh at present. FNCA is a very good platform to work together and to compare our results with each other and disseminate it within our country for better management of CERVIX and NPC patients.
China
CERVIX III has been a standard treatment in my hospital. The following year, we will increase new patients to CERVIX IV.
Due to the benefits of medical insurance, 80% - 90% patients can be accepted sufficient medical care. From last year, we began to treat patients with IMRT. So, we will enroll more patients into NPC-II. NPC III group follow the protocol.
Indonesia
In general protocol of FNCA (Cervical CA & NPC) are accepted and adopt as a part of our protocol. Handicap:
Protocol cervical cancer difficulty enroll in registration especially in Surabaya due to different policy with GYN doctor.
Financial problem especially with the chemotherapy because most patients (60%) come from low / social economic problems.
Not all other specialists know and accept FNCA protocol because they are many research.
Korea
At present,5 big hospitals are adopting our CERVIX-II protocol for treating locally advanced CERVIX cancer. They are very satisfied with treatment results obtained for the last 5 years.
CERVIX III protocol has been already adopted by several hospitals including Kyung-Hee University Hospital and Eul-Ji University Hospital. A lot of cases will be filed up in the following 2 years in anticipation of good treatment results.
Malaysia
CERVIX III protocol is included into our department's CERVIX Treatment protocol. The findings were presented at ICARO in April 2009.
NPC I protocol is accepted as our department's protocol. NPC II is a bit difficult due to the distance metastases that we observe in the NPC cases.
CERVIX IV modified protocol is feasible and will start recruiting cases.
The FNCA meeting is an important meeting for us as the results presented and the discussions that we have with participants have a direct influence on our treatment. FNCA is focusing on Asian patients and the treatment outcome is directly useful for our patients. Hence, we feel that the FNCA meetings have been extremely helpful in our country. Having results from studies done in Asia is more useful and beneficial to us compared to those done in western countries.
Philippines
The FNCA activities has a very strong impact on the use of radiotherapy in the management of cervical and NPC. The protocol which were used in the different activities of FNCA and now part of the current clinical practice in the management of cervical and NPC. Our participations in FNCA activities has also given us an opportunity to appreciate the difference and commonalities in the practice of oncology in this part of Asia. It has truly enriched our understanding of how these cancers can be approached. It is hoped that these cooperation actively will continue in view of these enormous benefits that it gave to the participating countries.
Thailand
CERVIX I is a standard treatment before chemotherapy era in my hospital.
CERVIX II has been adopted to a mini-research of one at my resident. After she finished her trainings, she invented SBDD herself and use it routinely in her hospital. She just submitted her large series
nation forum at research in Thailand and get best research award in 2009.
CERVIX III is a standard treatment, practice guideline and integrated in medical student and resident in Radiation Oncology Training.
NPC I and NPC II have been discussed and we cannot totally convince medical oncologist. They are still thinking I am afraid of distant metastases more than compliance, so they stick with INT0099 regimen.
Vietnam
CERVIX and NPC protocol became standardised protocol in our hospital. I can share information about latest knowledge not only with participants of FNCA but also with young staff in our hospital. Before attending of FNCA I was not very aware of CCRT in NPC and CERVIX. But through the annual FNCA meeting I am now able to convince my colleagues to adopt this protocol.
|
Program of
FNCA FY2009 Workshop on Radiation Oncology
January, 18(Mon)-21(Thu), 2010
Kuching, Sarawak, Malaysia
Organized by:
Ministry of Health, Malaysia
Ministry of Education, Culture, Sport, Science, and Technology (MEXT, Japan)
In cooperation with:
Nuclear Safety Research Association (NSRA)
Date: January 18-21, 2010
Venue & Stay: Riverside Majestic Hotel, Kuching, Sarawak, Malaysia
Sunday, January 17
Arrive at Kuching, Move to the Hotel
Day 1, Monday, January 18
| 08:30-09:00 |
Registration |
| 09:00-10:00 |
Opening Ceremony
Chair: Tang Tieng Swee (Malaysia)
Welcome Remark:
Dr. Zulkipli Jantan, Sarawak State Health Director
Ministry of Health, Malaysia
Remark:
Hirohiko Tsujii (Japan), Project Leader
Special Lecture: Current Status of Radiation Oncology in Malaysia
Tang Tieng Swee (Malaysia)
Adoption of the Agenda
Group Photograph
|
| 10:00-10:15 |
coffee break |
| 10:15-12:00 |
Session 1: Phase II Study of Chemoradiotherapy for Locally Advanced Cervical Cancer (CERVIX-III)
Co-chairs:
Kulllathorn Thephamongkhol (Thailand) (reporter)
Nana Supriana (Indonesia)
1) Presentation on the follow-up data from each country
China
Indonesia
Japan
Korea
Malaysia
The Philippines
Thailand
Viet Nam
2) Summary of the follow-up data
Shingo Kato (Japan)
3) Discussion
|
| 12:00-13:00 |
Lunch |
| 13:00-16:00 |
Session 2: Phase II Study of Concurrent Chemotherapy and Extended-Field Radiotherapy for Locally Advanced Cervical Cancer (CERVIX-IV)
Co-chairs:
Rey H. de los Reyes (the Philippines) (reporter)
Parvin A. Banu (Bangladesh)
1) Presentation on the clinical data from each country
Bangladesh
China
Indonesia
Japan
Korea
Malaysia
The Philippines
Thailand
2) Summary of the clinical data
Shingo Kato (Japan)
3) Discussion on the clinical data of Cervix-IV
4) Discussion on the next clinical trial
|
| 16:00-16:20 |
coffee break |
| 16:20-18:00 |
Session 3: QA/QC of External Beam Therapy
Co-chairs:
Tang Tieng Swee (Malaysia) (reporter)
Chul-Koo Cho (Korea)
1) QA/QC of External beam therapy, Report of the Fieldwork
Hideyuki Mizuno (Japan)
2) Schedule of the next Fieldwork
Hideyuki Mizuno (Japan)
3) Discussion
|
| 19:00-21:00 |
Welcome dinner
Hosted by MOH
|
Day 2, Tuesday, January 19
| 09:00-12:00 |
Session 4: Phase II Study of Chemoradiotherapy for NPC (any T N2-3) (NPC-I)
Co-chairs:
Beena Devi (Malaysia) (reporter)
Takehiro Inoue (Japan)
1) Presentation on the clinical data from each country
Bangladesh
China
Indonesia
Korea
Malaysia
The Philippines
Thailand
Viet Nam
2) Summary of the clinical data
Tatsuya Ohno (Japan)
3) Discussion
|
| 10:30-10:50 |
coffee break |
| 10:50-12:00 |
4) Evaluation of treatment results
Tatsuya Ohno (Japan)
5) Discussion
|
| 12:00-13:00 |
Lunch |
| 13:00-17:00 |
Session 5: Phase II Study of Chemoradiotherapy for NPC (T3-4 N0-1) (NPC-II)
Co-chairs:
Dang Huy Quoc Thinh (Viet Nam) (reporter)
Dyah Erawati (Indonesia)
1) Presentation on the clinical data from each country
Indonesia
Malaysia
Thailand
Viet Nam
2) Summary of the clinical data
Tatsuya Ohno (Japan)
3) Discussion
|
| 14:30-14:50 |
coffee break |
| 14:50-17:00 |
4) Evaluation of treatment results
Tatsuya Ohno (Japan)
5) Discussion on the clinical data of NPC-II
6) Discussion on the next clinical trial
|
| 18:00-20:00 |
Dinner
Hosted by Japanese members
|
Day 3, Wednesday, January 20
| 09:00-13:00 |
Session 6: Technical Visit at Radiotherapy Division Sarawak General Hospital |
| 13:00-14:00 |
Lunch |
| 14:00-17:00 |
Session 7: Future plan, Other activities
Co-chairs:
Hirohiko Tsujii (Japan)
Xu Xiaoting (China)
|
| 14:00-15:30 |
Next Clinical Trials for Cervical Cancer and NPC |
| 15:30-15:45 |
coffee break |
| 15:45-17:00 |
Next Workshop, others
Schedule of the next Workshop, Other activities
|
| 19:00-21:00 |
Dinner |
Day 4, Thursday, January 21
| 09:00-11:00 |
Session 8: Drafting the Workshop Minutes
Co-chairs:
Rey H. de los Reyes (the Philippines)
Chul-Koo Cho (Korea)
1) Discussion
2) Adoption of the Minutes
|
| 11:00-11:15 |
coffee break |
| 11:15-12:00 |
Closing Session
Closing Remark: Hirohiko Tsujii (Japan)
Remark:
Dr. Muhd Noor B. Muhd Yunus
FNCA Coordinator of Malaysia
Deputy Director General (Technical)
Malaysian Nuclear Agency (Nuclear Malaysia)
|
| 12:00-13:00 |
Lunch |
| 13:30-15:00 |
Session 9: Open Lecture
Co-Chairs:
Tang Tieng Swee (Malaysia)
C. R. Beena Devi (Malaysia)
Opening Remark: Hirohiko Tsujii (Japan), Project Leader
Welcome Address
Dr. Junaidi Diki, (Malaysia)
Director, Sarawak General Hospital
1) The Good, The Bad and The Unknown in Rheumatology
Teh Cheng Lay (Rheumatologist, Sarawak General Hospital, Malaysia)
2) Introduction of the FNCA Radiation Oncology Project
Shingo Kato (Japan)
3) Overview of Radiotherapy at Sarawak General Hospital
C. R. Beena Devi (Malaysia)
4) SBRT in treating extracranial tumors by using CyberKnife system
Chul-Koo Cho (Korea)
5) Concurrent Chemoradiotherapy for Cervical Cancer
Kulllathorn Thephamongkhol (Thailand)
6) Present Status and Future of Radiotherapy in Japan
Takehiro Inoue (Japan)
Closing Remark: Tang Tieng Swee (Malaysia)
|
|
List of Participants
FNCA FY2009 Workshop on Radiation Oncology
January, 18(Mon)-21(Thu), 2010
Kuching, Sarawak, Malaysia
Bangladesh
Dr. PARVIN AKHTER BANU
Senior Consultant,
Senior radiation oncologist, The cancer Unit
Delta Hospitals Limited
China
Dr. Xu Xiaoting
Doctor
The First Affiliated Hospital of Suzhou University
Indonesia
Dr. Nana Supriana
Medical Staff, Dept.Radiotherapy
Cipto Mangunkusumo Hospital
Dr. Dyah Erawati
Head of Radiotherapy Department, Divisin of Radiotherapy
Dr. Soetomo General Hospital
Japan
Dr. Hirohiko Tsujii
Executive Director
National Institute of Radiological Sciences
Dr. Takehiro Inoue
Professor
Osaka University, Graduate School of Medicine
Dr. Kunihiko Kobayashi
Professor, Department of Respiratory Med.
Saitama Medical University International Medical Center
Dr. Shingo Kato
Section Head, Clinical Diagnosis Section
Research Center Hospital for Charged Particle Therapy, National Institute of Radiological Sciences of Japan
Dr. Tatsuya Ohno
Associate Professor
Gunma University Heavy Ion Medical Center
Dr. Hideyuki Mizuno
Researcher (Medical Physicist)
National Institute of Radiological Sciences
Mr. Takehiko Kato
Project Manager, International Affairs and Research Association
Nuclear Safety Research Association (NSRA)
Korea
Dr. Chul-Koo Cho
Head, Department of Radiation Oncology
Korea Cancer Center Hospital
Malaysia
Dr. Tang Tieng Swee
Senior Medical Physicist, Department of Radiotherapy & Oncology
Sarawak General Hospital
Dr. C.R. Beena Devi
Senior Consultant Clinical Oncologist, Department of Radiotherapy & Oncology
Sarawak General Hospital
Philippines
Dr. Rey H. De los Reyes
Chairman, Department of Obsterrics and Gynecology
Jose R. Reyes Memorial Medical Center
Thailand
Dr. Kulllathorn Thephamongkhol
Lecturer
Siriraj Hospital, Mahidol University
Vietnam
Dr. DANG HUY QUOC THINH
Vice Director - Head of department of RT
Ho Chi Minh City Oncology Hospital
|
|
|