Report of
FNCA FY2020 Online Workshop on Radiation Oncology
November 27, 2020
Outline of Workshop
| i) Date : |
November 27th, 2020 |
| ii) Venue : |
ZOOM |
| iii) Host Organization : |
Ministry of Education, Culture, Sports, Science and Technology(MEXT) |
| iv) Participants : |
44 Bangladesh, China, Indonesia, Japan, Kazakhstan, Korea, Malaysia, Mongolia, Philippines, Thailand, Vietnam. |
The FNCA FY2020 Online Workshop on Radiation Oncology was held on November 27th, 2020. The meeting was organized by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) in cooperation with Nuclear Safety Research Association (NSRA). Representatives from 11 FNCA member countries, namely Bangladesh, China, Indonesia, Japan, Kazakhstan, Korea, Malaysia, Mongolia, The Philippines, Thailand and Vietnam participated in the workshop (WS).
This project carries out international joint clinical studies in order to establish treatment protocols for uterine cervix cancer, nasopharyngeal cancer and breast cancer, which affect large number of people in FNCA member countries, and finally to improve the technique of radiation oncology in the Asian region.
Opening Session
Prof. KATO Shingo, the Project Leader of Radiation Oncology Project opened the workshop with his opening remarks.
Mr. SUZUKI Tetsu, MEXT, Mr. WADA Tomoaki, FNCA Coordinator of Japan and Dr. NAMBA Hideki, FNCA Advisor of Japan gave their remarks respectively.
Prospective Observational Study of 3D-Image guided brachy therapy for Locally Advanced Cervical Cancer (CERVIX-V)
This is a new protocol for cervical cancers following Cervix-IV. The patient enrollment started in 2018.
60 patients were enrolled into Cervix-V in total. The number of patients by county is
Bangladesh (1), China (7), Indonesia (9), Japan (5), Kazakhstan (6), Korea (0), Malaysia (10), Mongolia (2), Philippines (8) Thailand (12) and Vietnam (0).
Out of 60 patients, 54 patients were eligible.
As preliminary analysis of Cervix-V, 42 patients whose follow-up periods exceeded 6 months were analyzed.
All patients were treated with 3D-IGBT.
Compared to the reference doses, almost all cases satisfied those doses.
-39 (93%) patients achieved adequate doses for HR-CTV D90.
-41 (98%) patients achieved the dose constraint for bladder D2cc
-41 (98%) patients achieved the dose constraint for rectum D2cc.
-40 (95%) patients achieved the dose constraint for sigmoid colon D2cc.
-Grade 3 acute hematological toxicity was observed in 5 (12%) patients. These toxicities were manageable.
-Grade 3 or worse acute non-hematological toxicity was observed in 1 (2%) patients so far.
-Grade 3 or worse late toxicity has not been observed so far.
-The 2-year overall survival (OS), local control (LC), and disease free survival (DFS) were 91%, 88%, and 72%, respectively.
Patient enrollment into Cervix-V goes well. Members were encouraged to continue to enroll patients.
Phase II Study of Neoadjuvant Chemotherapy with Concurrent Chemoradiotherapy (CCRT) for Nasopharyngeal Carcinoma (NPC-III)
A total 120 patients have been registered in this protocol.
The number of patients by county is Bangladesh (1), China (9), Indonesia (12), Japan (0), Kazakhstan (0), Korea (0), Malaysia (31), Mongolia (0), Philippines (7) Thailand (0) and Vietnam (60).
Japan presented the summary of analyzed clinical data of NPC-III. The Summary are as follows.
- Patient enrollment was completed in 2019.
- Current Total number of patients enrolled in NPC-III is 120.
- Median follow up period is 38 months.
- Some follow up data with acute toxicities enrolled in 2019 has not been submitted yet.
-The treatment results match with NPC-I (CCRT and adjuvant chemotherapy). However, there are significant differences as well. Locoregional control of NPC-III (Induction chemotherapy and CCRT) is lower than NPC-I while overall survival is better. These results are preliminary results.
The primary endpoint of this clinical trial is 3-year OS. The patients need to be followed up for another 2 years. The 3 year follow up results will be the written preliminary report.

Phase II Study of Hypofractionated Radiotherapy for Breast Cancer (Postmastectomy Radiation Therapy (PMRT)/BREAST-I)
The total number of registered PMRT patients was 222 (Bangladesh (84), China (13), Indonesia (0), Japan (15), Kazakhstan (20), Korea (0), Malaysia (0), Mongolia (26), Philippines (18), Thailand (0) and Vietnam (46)).
Japan presented the summary of analyzed clinical data of PMRT/BREAST-I. The Summary is as below.
- 222 cases have been enrolled and analyzed while the target accrual number is 200.
- 1 patient's data that has not been analyzed after enrollment was newly added this year.
- 15% of grade 2 and over acute dermatitis and 1% of grade 2 subcutaneous acute toxicity have been observed.
- No grade 3 or over late toxicity has been observed.
- The 3-year locoregional control, progression free survival rates are 96.9%, 88.9%, respectively.
-Co-researchers in this clinical trial should grade toxicity correctly.
Participants agreed to write a report on acute toxicity of this protocol at this point.
The enrolment of patients into this protocol was completed in 2019 and the primary endpoint of the protocol is 5-year local recurrence free survival. Participants were requested to follow up the patients for 4 more years.
Phase II Study of Hypofractionated Radiotherapy for Breast Cancer (Whole Breast Irradiation(WBI)/BREST-I)
The total number of registered WBI patients was 227 (228 breast lesions). The number by country is Bangladesh (31), China (6), Indonesia (16), Japan (134), Kazakhstan (14), Korea (9), Malaysia (0), Mongolia (3), Philippines (0), Thailand (14) and Vietnam (0). Total number of WBI patients was 229. (229 patients / 230 breast lesions).
The summary of the clinical data is as follows.
Japan presented the summary of analyzed clinical data of WBI/BREAST-I. The Summary is as below.
-227 cases were enrolled in HF-WBI protocol from February 2013 until October 2018.
-227 patients with 228 tumors who completed the treatment were analyzed.
-13% of grade 2-3 acute dermatitis had been observed.
-1 locoregional recurrence, 4 distant metastases and 3 breast cancer death have been observed.
-No grade 3 or over late toxicity has been observed.
-The 3 year locoregional control, progression free survival rates are 99.6%, 98.6% respectively.
-Co-researchers in this clinical trial should grade toxicity correctly.
WS Participants agreed to write a report on acute toxicity of this protocol at this point.
Patient enrolment into this protocol was completed in 2018. Participants were requested to follow up the patients for 3 more years to assess the efficacy.
Breast-I clinical trials (PMRT and WBI) have been conducted very well so far.
QA/QC for 3D-IGBT
This activity aims to put in place reliable dosimetry in the institutes among the member countries for effective joint clinical studies. The audits in QA/QC of dosimetry measurement and radiation calibration have been conducted, which is for the reliable radiotherapy.
Dosimetry audit on 3D-IGBT by medical physicists started in 2019 along with CERVIX-V.
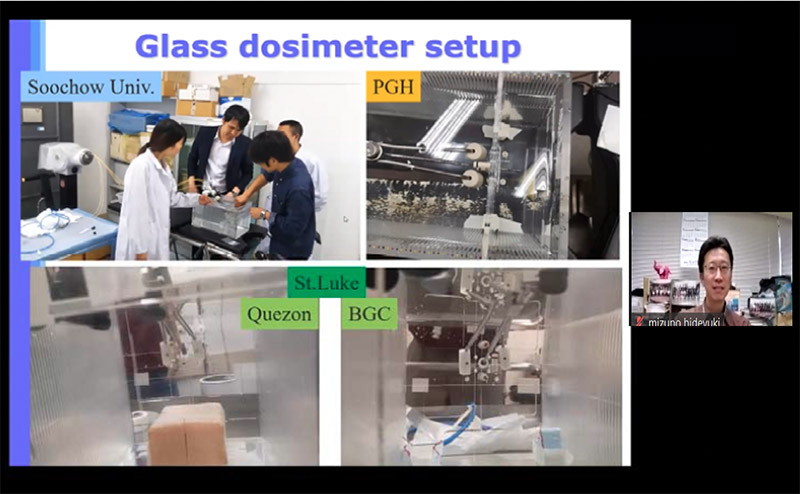
Japan reported the results of on-site audit for 3D-IGBT of the 4 hospitals namely the First Affiliated Hospital of Soochow University (China, October 2019) and Philippine General Hospital, St. Luke's Medical Center (Quezon City and Global City) (The Philippines, January 2020). Main points from his report are as follows.
-The dosimetry audits in the 4 hospitals were successfully conducted.
-Measured dose for point A, bladder and rectum were agreed with TPS calculated value within a tolerance level for all.
-Applicator offset value was measured for the centers and 1 hospital showed 2 mm difference between measured and stated value. The hospital staff re-measured the value after the on-site audit and confirmed the consistency with the audit results and modified it. Finally offset data became consistent for all cases.
- Measured source strength and treatment planning system (TPS) registered value agreed within a tolerance level for all hospitals.
The on-site audit is being suspended by the COVID-19 impact. The audit will resume after current difficult situation is improved.
Special Topic -Status of Radiotherapies during the COVID-19 Crisis in FNCA Member Countries-
The purpose of this special session was to know and also share the status of radiotherapies during the COVID-19 in FNCA member countries.
15 hospitals' cases (from each FNCA country) were introduced.
Presenters introduced national data and statistics regarding COVID-19 and explained on their efforts and strategies to perform radiotherapies avoiding infection risk.
Participants learnt there are many ways to reduce the infection risk of patients and medical staffs. As mentioned in presentations, hypo-fractionated radiotherapy should be used more actively and frequently. Safe and effective hypo-fractionation treatment regimen including palliative radiotherapy should be established among FNCA countries.
Future Plans
1) New Clinical Trials / Palliative Radiation Therapy for Bone Metastasis & Brain Metastasis
In 2019's WS, radiotherapies for bone metastasis and brain metastasis were proposed and discussed as new clinical trials. To draft those protocols, questionnaires were conducted among FNCA members prior to WS.
Dr. MAKISHIMA Hirokazu (Japan)introduced the results of questionnaire on brain metastasis. He also introduced the outline of the clinical trial and draft protocol.
Discussion points of the draft protocol are additional secondary outcomes, sample size, inclusion/exclusion criteria and evaluation method of efficacy. Members can exchange their comments and requests on those points by emails afterwards.
Dr. Kullathorn Thephamongkhol (Thailand) also introduced briefly the results of the questionnaire on radiotherapies for brain metastasis. He summarized the answers regarding "environment of palliative treatment" and "case management" in FNCA members' hospitals.
Based on the questionnaire results, he suggested several potential protocols e.g. single arm study as practice (QoL Study), randomized control trial (RCT) to resolve variation, prospective study for technology transfer, retrospective study to predictive survival benefit /avoidance of unnecessary whole brain radiation therapy (WBRT).
WS participants will exchange emails on the 2 new clinical trials for further discussion after WS.
2) 2021's Workshop
2021's workshop will be held in Mongolia in the beginning of September if the spread of COVID-19 infections has settled down.
|