Report of
FNCA2008 Workshop
on Cyclotron and Positron Emission Tomography (PET) in Medicine
Jan. 6-9, 2009 Kuala Lumpur, Malaysia
Background:
The third FNCA Workshop on Cyclotron and PET in Medicine was held from January 6 to 9, 2009, at Kuala Lumpur that is the capital city of Lead Country of this project, Malaysia. It was jointly organized by Malaysian Nuclear Agency (Nuclear Malaysia), Ministry of Science, Technology and Innovation, Malaysia (MOSTI) and Ministry of Education, Culture, Sports, Science & Technology of Japan (MEXT). The workshop was attended by delegates from 7 FNCA countries namely People's Republic of China, Indonesia, Japan, Malaysia, The Philippines, Thailand and Vietnam. The participants of workshop amounted to 29 persons.
The objectives of this project are
- Improvement of diagnostic technique for nuclear medicine in Asian countries.
- Early detection with advanced technology such as PET and PET/CT, contributes to human health in Asia.
The themes of three areas namely “Imaging instrumentation”, “Radiation safety and PET radiopharmaceuticals” and “Clinical diagnosis” were considered through their small-group discussion respectively at the workshop.
 |
| Participants of Workshop |
Group 1 - Imaging Instrumentation
The theme for Group 1 is radiation safety, quality assurance and quality control for PET/CT imaging. The activity and target outcome that is expected through group discussions are the establishment of radiation protection and performance evaluation of PET/CT.
Group members drafted the guideline on the performance evaluation of PET scanner and CT scanner, named “guideline for radiation protection and performance evaluation of PET-CT imaging equipment”. This document is expected to be utilized effectively for the improvement of QA/QC in the developing countries.
Group 2 - Radiation Safety and PET Radiopharmaceuticals
The theme for Group 2 is the QA/QC for PET radiopharmaceuticals, radiation safety for cyclotron and production of 18F-FDG. The activity and target outcome that is expected through group discussions are the establishment of guidelines for the cyclotron performance, 18F-FDG production and QA/QC for 18F-FDG.
Group members discussed about the contents of the guideline. In the result, they agreed that Malaysian representatives were going to finalize the guideline based on the Japanese reference of the production and QA/QC of FDG, combining the conditions of member countries.
Group 3 - Clinical Diagnosis
Group 3 has the theme of publication of case review in clinical PET and thus the group hopes to introduce the FNCA case review system for clinical PET.
They expect the FNCA case review system will be utilized for not only the education item of relevant parties but also the one promoting better understanding of the availability of PET among peoples.
Therefore, they decided in the workshop to add the academic description to every case for the better utilization of users.
The collection of the PET data was continued after the workshop and finally achieved the target value, 100 cases in March, 2009.
 |
 |
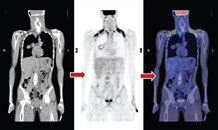
| Figure a : PET-CT shows an intense brown fat uptake |
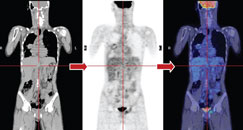
Figure b: Repeat PET-CT - no brown fat uptake over the neck region after the administration of muscle relaxant |
Recommendations
Members of the 3rd FNCA workshop on the Applications of Cyclotron and PET in Medicine have agreed on the following recommendations:
This FNCA project to be extended to include further expansion of the existing scope.
To include preparing the guideline for radiation safety aspect in Cyclotron and PET radiopharmaceutical production.
To include pre-installation and acceptance criteria for Cyclotron.
To develop the Quality Assurance guidelines in all sub groups.
To carry out surveillance on personal exposure dose in PET/CT and Cyclotron facilities for optimization.
Open seminar
Open Seminar was held from January 8 to 9, 2009.
The authorities from Malaysia made 10 presentations, China made 2, and Japan made 3 on the several phases of the nuclear medicine including advanced PET technology and shared a lot of information with the audience.
There were almost one hundred participants including lectures at this event.
 |
 |
| Participants of Open Seminar |
Seine of Open Seminar |
|